

Protonated form is nothing but the ionized form while unprotonated form is the unionized form.

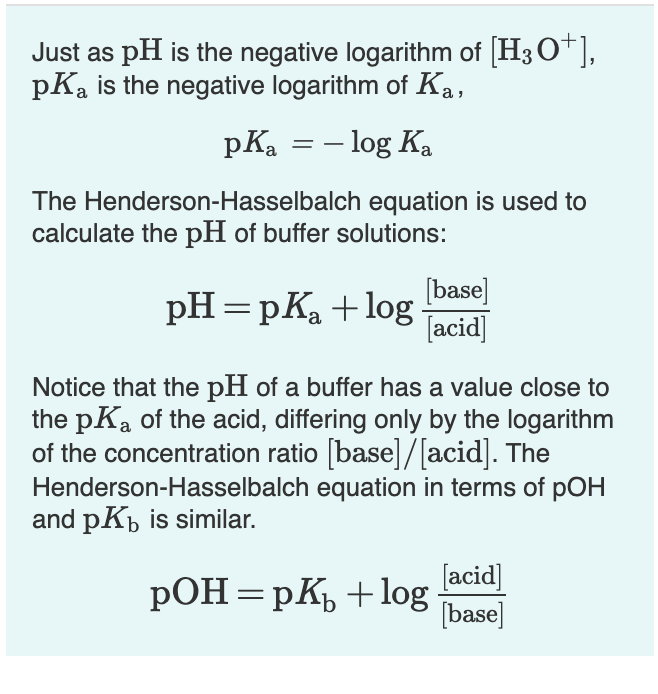
But here the ratio of protonated to unprotonated forms of the drug was asked. Morphine is an opioid analgesic that has tertiary amine in the ring hence acts as weak base. Working Example 3Ĭalculate the ratio of protonated to unprotonated forms of morphine at pH 5. Here it is a weak base, so we have to apply the following formula.įinally we conclude with a different example. Working Example 2Ĭalculate the fraction of drug in the ionized form at pH 7.7 of a tissue for a basic drug with pKa 8.7. Since it is a weakly acidic drug, let’s apply the following formula. Now let’s go with few of the practical examples and let’s see how can we solve them.Ĭalculate percentage ionized of a weakly acidic drug at a pH of 4.6 with pKa value as 8.6. So the percentage ionization will beĪlternatively, we can also rearrange the above equation by reversing the term pH-pKa into pKa-pH where we get the formula as Here the portion of unionized form is 10 (pH – pKa) whereas ionized form is 1. Now the ratio of unionized to ionized forms of the base can be written as Now writing Hendersen-Hasselbalch equation for the conjugate acid Now we can write chemical equilibrium for the conjugate acid as below. Similarly we can obtain the formula for a weak base like B whose conjugate acid is BH +. From this we can calculate the percent ionization. Now, we know the ratio of ionized form to unionized form. Now let’s rearrange this equation so that we will get the ratio of ionized to unionized forms of the weak acid. Where A - is the ionized form the weak acid whereas HA is the unionized form. When it dissolved in water, it is not completely ionised but achieves equilibrium so that it can exist both in ionized as well as unionised forms.īy applying Hendersen-Hasselbach equation for the weak acid, we can relate the pH of the solution with the pKa of the drug and its ionization. Let’s take a weak electrolyte such as HA which acts like weak acid. Here let’s see how we can calculate it along with few examples. So, calculation of percent ionization is an essential task we come across many times while we deal with pharmacokinetics of drugs.


 0 kommentar(er)
0 kommentar(er)
